Manufacturing Facility
At Tridev Biosciences, we are committed to delivering high-quality pharmaceutical solutions through state-of-the-art manufacturing and rigorous quality standards.
Located in the heart of Baddi, Himachal Pradesh, we specialize in the production of tablets and capsules, catering to third-party manufacturers and fulfilling government tenders.
With certifications like ISO 9001:2015, GMP, and GLP, we ensure that every product meets stringent industry benchmarks. Backed by a fully equipped Quality Control (QC) laboratory, we uphold excellence at every step of the manufacturing process.
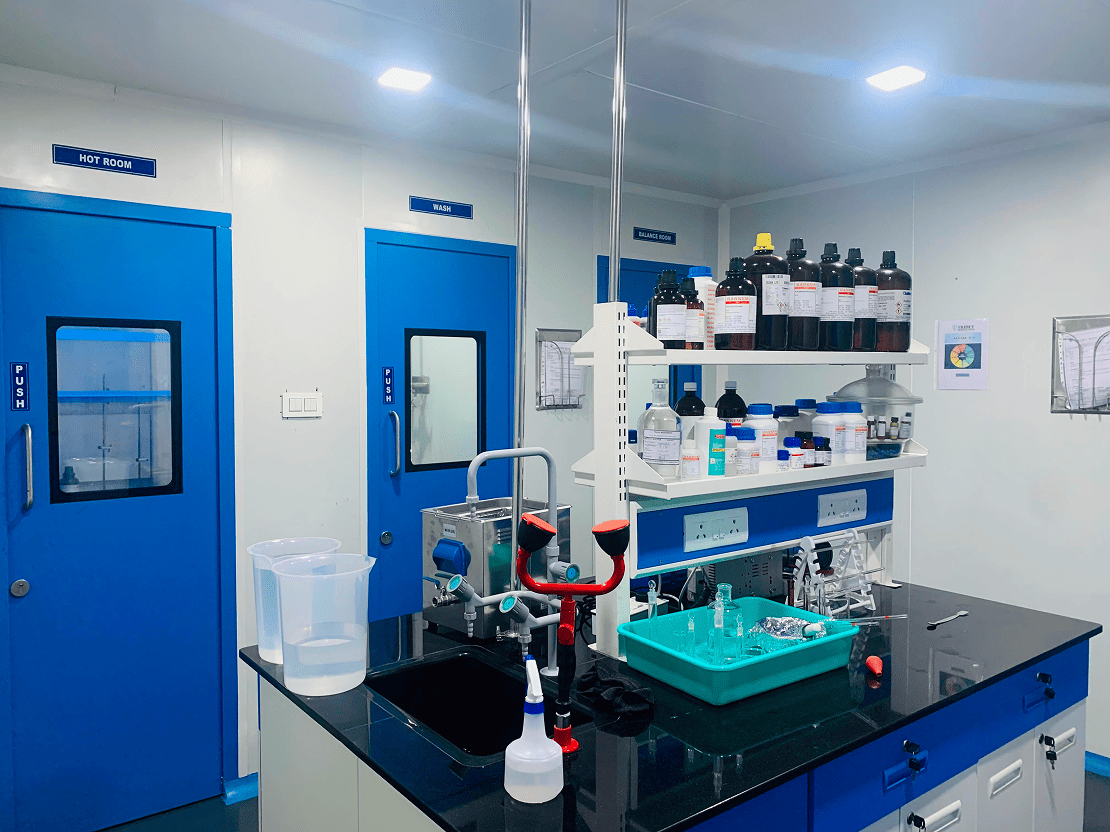
Equipped for Precision: Our Quality Control Lab
Our state-of-the-art Quality Control Lab is equipped with advanced instruments such as HPLC, UV-Visible Spectrophotometer, Dissolution and Disintegration Apparatus, Friability Apparatus and many more. These tools enable us to conduct precise testing and analysis to ensure every product meets the highest standards of quality, safety, and regulatory compliance.
From Raw Material to Ready Product: Our Manufacturing Workflow
Our manufacturing process follows a streamlined, quality-driven approach—from sourcing certified raw materials to delivering finished pharmaceutical products. Each step is guided by strict compliance with GMP and GLP standards, ensuring precision, safety, and consistency in every batch.



Testimonial
Bhagyashree Kothawade
Rajguru Chemist
Dnyaneshwar Nankar
Frequently Asked Questions
What types of products do you manufacture?
We manufacture a wide range of pharmaceutical products including tablets and capsules
How do you ensure product quality and consistency?
Our in-house QC & QA teams conduct rigorous testing at every stage—from raw material procurement to final product release.
What is your minimum order quantity (MOQ)?
MOQ varies based on the product and formulation. Please contact our team to discuss specific requirements and custom batch sizes.
How do you manage regulatory compliance?
Our processes are designed to comply with WHO, FDA guidelines, supported by robust documentation, validation, and audit readiness.
What kind of testing facilities do you have in-house?
We have a fully equipped Quality Control lab for physical, chemical, and microbial testing, including HPLC, UV spectrophotometer, dissolution testing, and stability chambers.
Do you offer third-party or contract manufacturing services?
Absolutely. We provide contract manufacturing and private label solutions to pharmaceutical companies looking for reliable, high-quality production.
How can I get a quote/start a partnership with Tridev Biosciences?
Simply reach out to us via our contact form, email, or phone. Our business development team will guide you through the process and provide a tailored solution.
Can I visit your manufacturing facility?
Yes, we welcome facility visits for our partners and clients. Visits can be scheduled with prior notice and proper safety protocols in place.